
Templates Community /
Aluminum Extraction
Aluminum Extraction
Captain O Captain
Published on 2021-03-17

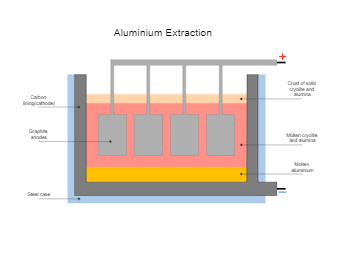
Aluminium Extraction is done by electrolysis, but first the aluminum oxide must be melted so that electricity can pass through it. As shown in the attached Aluminum Extraction diagram, aluminum oxide has a very high melting point (over 2,000 C) so it would be expensive to melt it. The use of molten cryolite as a solvent reduces some of the energy costs involved in extracting aluminum by allowing the ions in aluminum oxide to move freely at a lower temperature. In the Aluminum Extraction diagram, we have a steel case where molten aluminum is present along with Graphite anodes that are used for the Aluminum Extraction. The extraction process is accelerated and improved by using molten cryolite.
Tag
science
Share
Report
2
294

Post
Recommended Templates
Loading
