
Templates Community /
Carbon Dioxide Properties
Carbon Dioxide Properties
Kiraaaa
Published on 2021-03-30

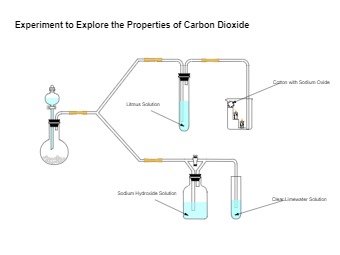
Carbon dioxide is a colorless gas with a density about 53 percent higher than that of dry air. Carbon Dioxide (CO2) occurs naturally in Earth’s atmosphere as a trace gas. Since CO2 is soluble in water, it occurs naturally in groundwater, rivers and lakes, glaciers, and seawater. The carbon dioxide molecule is linear and centrosymmetric at equilibrium. As the diagram suggests, carbon dioxide is used as a refrigerant, in fire extinguishers, for inflating life rafts and life jackets, foaming rubber and plastics, immobilizing animals before slaughter, and in carbonated beverages. Carbon dioxide has no liquid state at a pressure below 5.1 standard atmospheres. At 1 atmosphere, the CO2 deposits directly to a solid at a temperature below -78.5 degrees C.
Tag
Chemistry Drawing
Share
Report
4
296

Post
Recommended Templates
Loading
