
Templates Community /
Chemical Reaction Type
Chemical Reaction Type
easy diagrams
Published on 2021-04-01

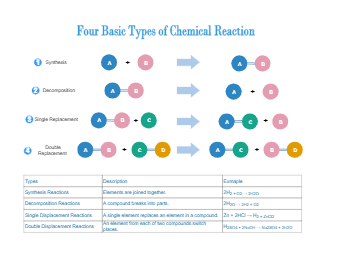
A chemical reaction is in which the chemical bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance. The chemical reaction diagram shows different types of chemical reactions. As illustrated in the diagram, a synthesis reaction is a type of reaction in which multiple reactants combine to form a single product. A decomposition reaction is a reaction in which a compound breaks down into two or simpler substances. Also, a single displacement reaction is a reaction in which one element is substituted for another element in a compound. Lastly, a double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products.
Tag
Chemistry Drawing
Share
Report
2
526

Post
Recommended Templates
Loading
