
UREA
KIM RITCHELE PADUA
Published on 2021-12-15

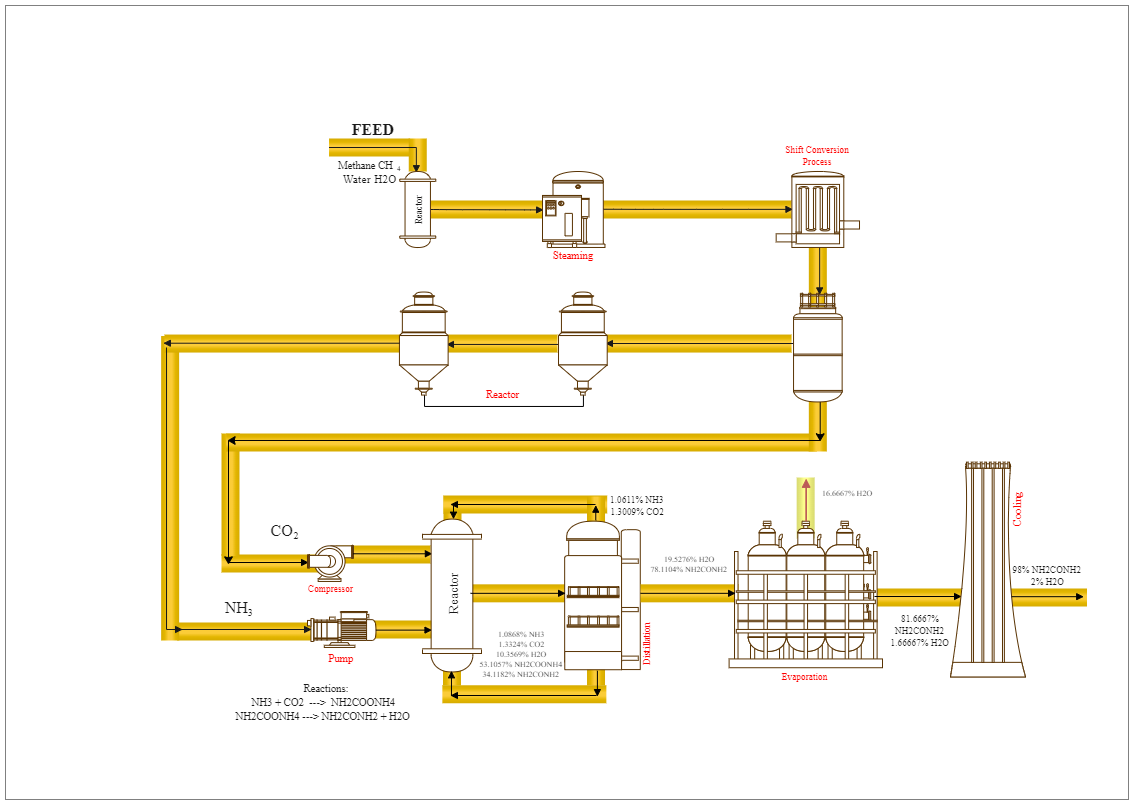
The urea contains unreacted NH3 and CO2, and ammonium carbamate. As the pressure is reduced and heat applied, the NH2COONH4 decomposes to NH3 and CO2. Urea forms when dietary proteins make amino acids after digestion. The liver breaks down excess amino acids to make ammonia, then converts this into urea, which is less toxic in the body than ammonia. As the reaction diagram suggests, the reaction by which urea decomposes has been studied extensively over the past century. Urea decomposition yields cyanate and ammonium ions in an aqueous solution, (NH2)2CO → CNO− + NH4+. An elimination mechanism appears to be operative. Cyanate ion further readily undergoes conversion to CO2 and ammonia.
Tag
diagram
Share
Report
0
146

Post
Recommended Templates
Loading
