
Templates Community /
Osmosis Diagram
Osmosis Diagram
Community Helper
Published on 2022-03-03

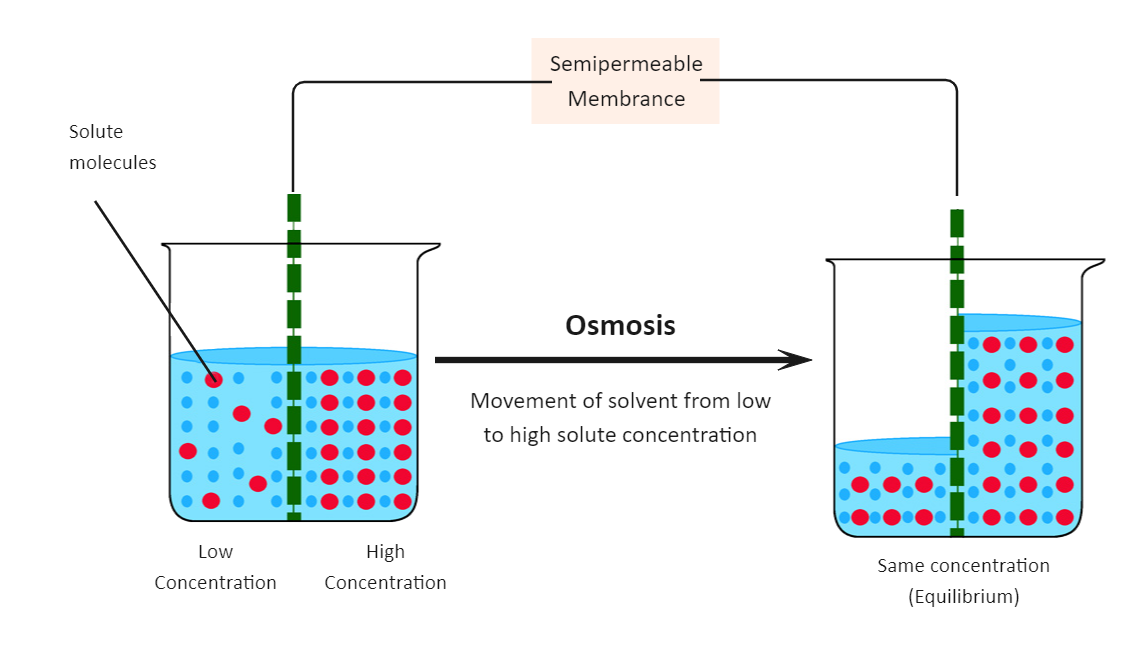
Before we learn about the following osmosis diagram, let us first understand the real meaning behind Osmosis. As taught in the junior classes, Osmosis is the diffusion of water across a partially permeable membrane from a dilute solution (high concentration of water) to a concentrated solution (low concentration of water). As we see in the following osmosis diagram, the sugar concentration is initially higher on the right side of the membrane. Therefore, water moves by Osmosis to the right-hand side to equalize the concentrations. In the following osmosis diagram, we have shown how the movement of solvent from low to high solute concentration occurs.
Tag
science diagram
Share
Report
3
407

Post
Recommended Templates
Loading
