
Templates Community /
Adverse Event Flow Chart
Adverse Event Flow Chart
Community Helper
Published on 2022-09-20

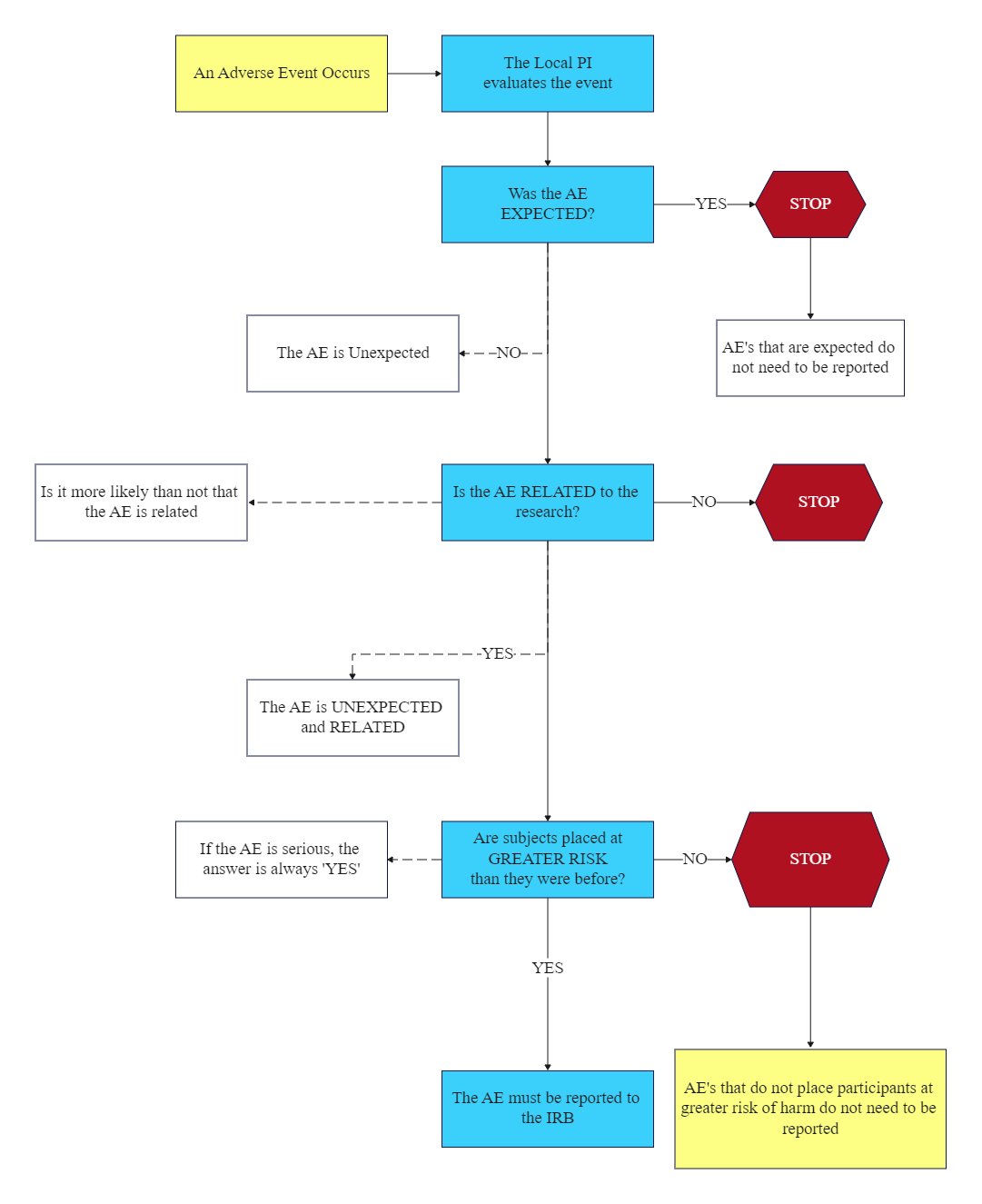
The advent event analysis is depicted in this Adverse Event Flow Chart. There are numerous methods for detecting adverse events, including reporting systems, document review, automated clinical data surveillance, and patient progress monitoring. These complementary approaches necessitate diverse data elements such as demographic information, signs and symptoms, medications, test results, diagnoses, therapies, and outcomes. While all available methods are complementary and have strengths and weaknesses, automated surveillance will likely become the primary source of adverse event data. More research is required to improve these detection systems' effectiveness and broaden the types of adverse events detected using automated triggers, as illustrated in this Adverse Event Flow Chart.
Tag
Event Flow Diagram
flowchart
Share
Report
0
275

Post
Recommended Templates
Loading
