A Guide to Understand Chlorine with Diagram
Chlorine is an oxidizing agent and plays an essential role in modern industries. The students must have ideas about chlorine production and its availability in nature. Thus, they should know about the chlorine properties and their reactivity. They should know the chemical and physical properties of chlorine thoroughly. It will help the students to learn about its water solubility, valency, and other things smoothly.
1. The Chlorine
Chlorine is a toxic gas discovered by Carl Wilhelm Scheel in 1774. The name Chlorine comes from the Greek word ‘Chloros,’ which means greenish-yellow. Chlorine gas has a yellowish-green color and a pungent smell which creates a choking effect. In the periodic table, it is the 3rd period and p block with the atomic number 17. Chlorine is mainly used as a disinfectant. Other than that, water treatment plants, the textile industry, and paint manufacturers use this gas. Chlorine gas helps in making PVC for water pipes, electric wires, etcetera.
In Organic chemistry, chlorine is an oxidizing agent. It is poisonous, and hence many countries during the World war used chlorine as a chemical weapon.
Chlorine is there in anesthetics like chloroform which causes liver damage when used excessively. Chlorine ion is crucial for the human body because it is present in cell fluid and extracellular fluids for ion balancing. The human body also gets chlorine from salt (NaCl). However, free chlorine is not available in nature. It comes from Halite or sodium chloride.
About 40 million tonnes of chlorine is produced annually in brine or sodium chloride solution. Other than that, Halite, Carnallite (magnesium potassium chloride), and sylvite (potassium chloride) also have some chlorine.

1.1 The chemical properties of chlorine
The chemical properties of an element determine its reactivity with other elements and changes that can occur due to burning, exploding, and more. Here are the chlorine chemical properties (Cl):
- Chlorine is immensely corrosive and toxic. It produces negative ions and is a powerful oxidizing agent.
- Reaction with metals: For most metals, chlorine is flammable above a specific temperature, especially during their reaction with dry chlorine. For example, Steel catches fire at 300℉ while reacting with dry chlorine. Chlorine is highly corrosive to most metals due to the property of the formation of HCl.
- Reaction with water: Chlorine is slightly water-soluble, but its solubility in water mostly depends on the water temperature. (0.3-0.7%).
- Reaction with Organic compounds: When chlorine reacts with organic compounds, it produces derivatives. Reactions of chlorine with alcohol, ethers, and hydrocarbons may be violent sometimes, and hence the procedure should be conducted within a controlled environment of the laboratory. Students should take much-needed precautions if they are experimenting with it in plants.
- Combustion and explosion of Chlorine: If a small amount of moisture is present, the alkali metals react with chlorine by the process of combustion. When hydrogen and chlorine are mixed at a specific rate, it may result in an explosion.
- Oxidation: Chlorine can form hypochlorites, chlorites, chlorates, and different oxides - Cl2O, ClO2, Cl2O6, Cl2O7, Cl2O8, and more.
- Valency: Though for Chlorine the valency in -1, it can also participate in reaction with valence of +1, +2, +3, +4, +5, +7.
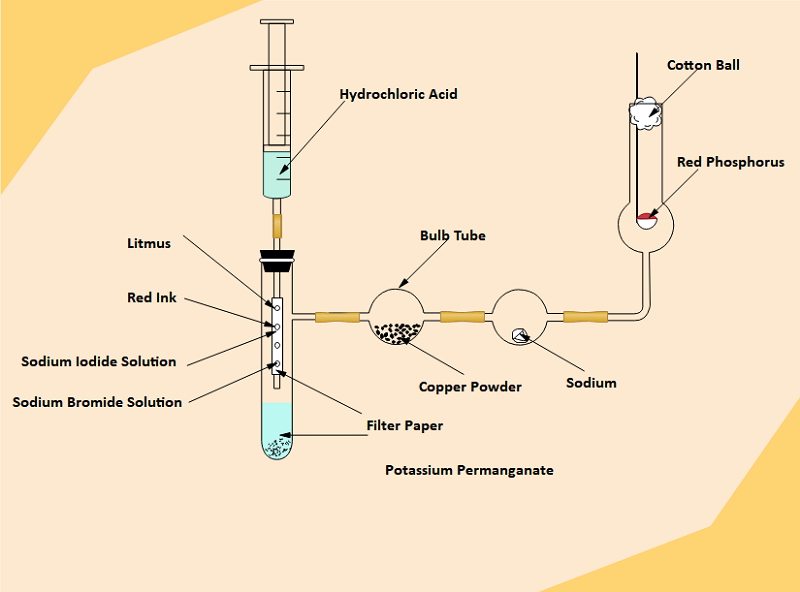 Source:EdrawMax Online
Source:EdrawMax Online
1.2 The physical properties of chlorine
Students can decipher the physical properties of an element without subjecting it to any reaction or change. Besides the chemical properties, the students must also know the physical properties to understand their nature.
Here are the physical properties of chlorine:
- Chlorine is a yellowish-green gas with a pungent and suffocating odor.
- The gas is dense and is almost 2.5 times heavier than air.
- Chlorine is water-soluble, but it depends on the temperature of the water as well. But there is an aqueous solution of chlorine formed with chlorine, hydrochloric acid, and hypochlorous acid.
- Melting point is the temperature, which is required for an element to transform from solid to liquid. The point of temperature at which the liquid turns into a gas is known as the boiling point. Chlorine is a gas at normal temperature. Therefore, it has a pretty low melting point. Hence, its melting point -34℃, and its boiling point is -100℃.
- The specific gravity of chlorine in the gaseous state is 2.48, while the specific gravity of chlorine in the liquid form is 1.46.
2. How to Draw the Chlorine Experiment Diagram?
It is also essential to know the production process of the element to learn the properties of chlorine. The students can use a chlorine experiment diagram for a better understanding of the process. They may create a chlorine experiment diagram by hand, but the process is lengthy and challenging.
2.1 How to Create Chlorine Diagram from Sketch
Here are the steps to draw a chlorine experiment diagram by hand:
Step 1: To start with the diagram, the students first need to draw a straight line signifying the floor. Then on it, create a Bunsen burner and a stand. Now, place a flask on it. The students have to draw dotted lines at the bottom part of the flask, representing the mixture of MnO2 and NaCl. There is a funnel to pour sulfuric acid into the flask, and the funnel reaches the liquid present in the flask.
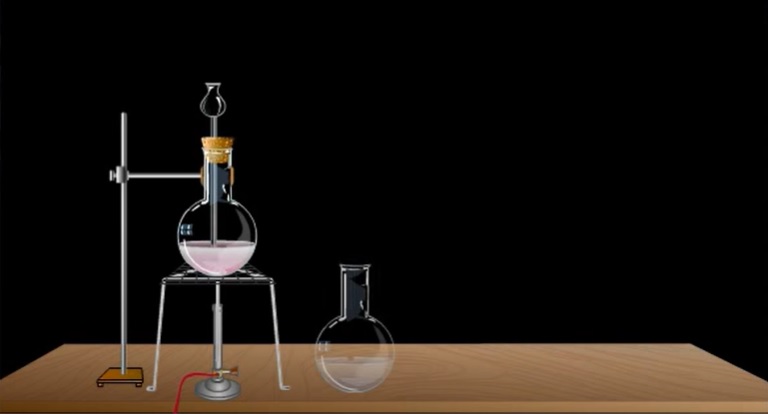
Step 2: Then the students have to draw another pipe opening in the flask and going all the way to another jar filled with water. The pipe should open inside the water. The jaw of water has another opening at the cap, leading to another similar jaw filled with sulphuric acid.
Step 3: Then at the end, there is a gas jar connected to the pipe of the second jar. This gas jar will collect chlorine at the end of the whole experiment.
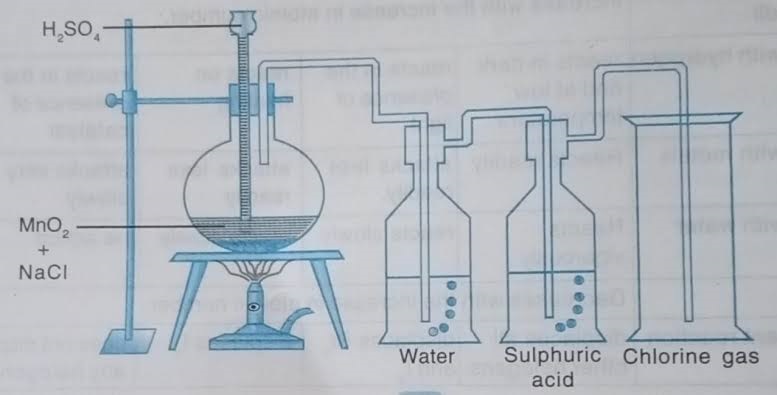
2.2 How to Create Chlorine Diagram Online
The process of creating this diagram by hand is challenging and time-taking. Therefore, the students can use EdrawMax Online tool, which is user-friendly. They can help the students to make a high-quality chlorine experiment diagram. The students can use the diagram to learn the processing and properties of chlorine gas. Here are a few simple steps which the students can follow to create their diagram on EdrawMax online tool:
Step 1: EdrawMax comes with an easy-to-use interface, making it easier for the students to work on the tool without prior experience. To start the process, they can open it and then open New. They have to click on the Science and Education tab, which comes with multiple education-related diagrams for the students who can use them in their lessons and projects.
Step 2: Under this option, the students or the users can find Chemistry. They can look for the chlorine experiment diagram. They can also be able to find several high-quality chemistry-related templates. These templates are beneficial for the students. They can use them in their dissertation papers and lessons.
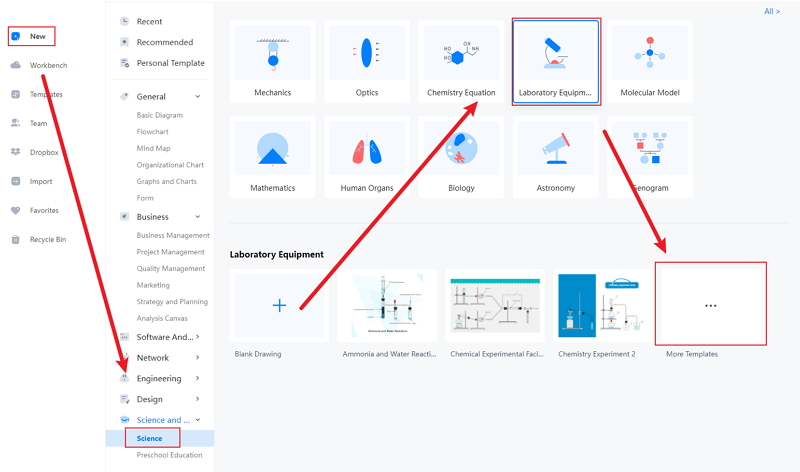
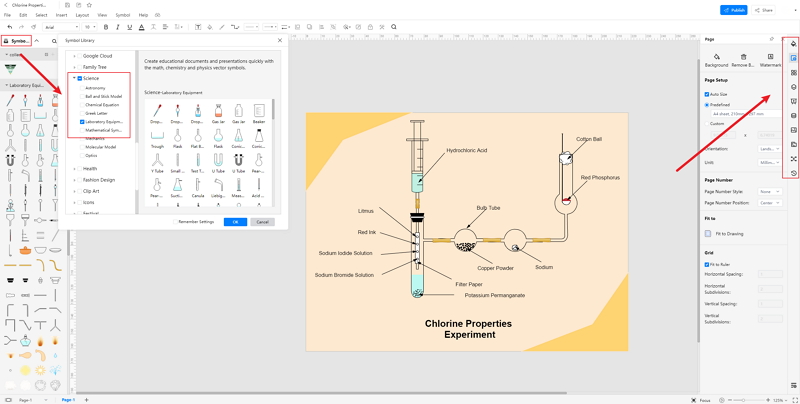
Step 3: Once the students have selected their required diagram, they can modify the same per their choice.
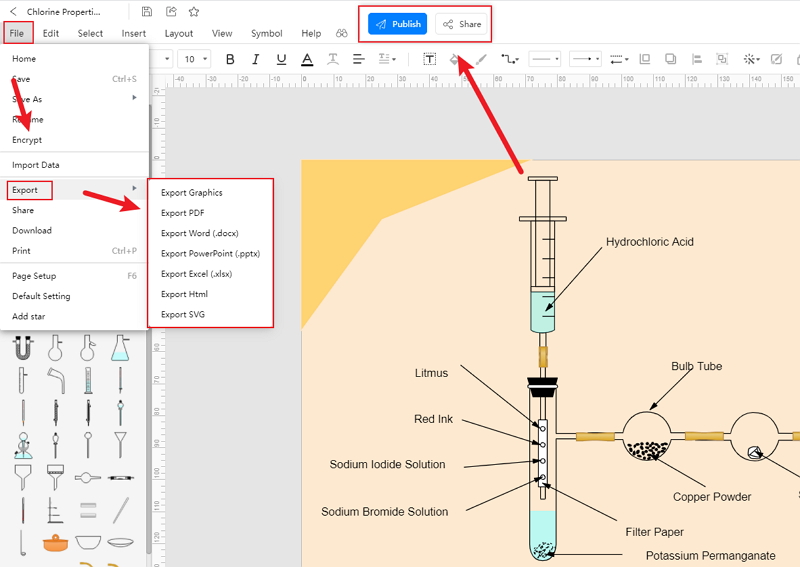
Step 4: After that, they can save it in multiple file formats and export it to use the same in the future.
3. The Effects of Chlorine
Effects on Human: Chlorine is highly reactive, and hence it is naturally not present in the human body. Humans can get exposed to it via contaminated water or air.
If the presence of chlorine is higher than normal in the air, it can have adverse reactions to the respiratory system of a human being. For example, the chlorine plant workers may suffer from chest pain, tightness, and breathing difficulty. If a high proportion of chlorine is mixed in the water of a swimming pool, it can harm the eyes and skin of the swimmers.
Effects on Environment: Chlorine gas directly does not harm the environment itself at a high rate. However, there are some indirect harms. Since chlorine is water-soluble, it can form chlorine compounds which will eventually get mixed with the underground water. It pollutes the underground water, and at the same time, can be harmful to the organisms that live in the water and soils contaminated with chlorine compounds. If animals have continuous exposure to chlorine in the air, that can affect their heart, immune system, and blood.
4. Conclusion
The students must learn about chlorine if they want to understand organic chemistry. They should have an idea about the properties of chlorine and the reactivity of the element. While learning, the students may use a chlorine experiment diagram. However, it is challenging to create a chlorine experiment diagram by hand. To avoid difficulty, the students must use the EdrawMax Online tool, which can help them draw the best quality chlorine experiment diagram.
In conclusion, EdrawMax Online is a quick-start diagramming tool, which is easier to make Chlorine diagram and any 280 types of diagrams. Also, it contains substantial built-in templates that you can use for free, or share your science diagrams with others in our template community.




